Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
How many significant digits are there in the following number: 15.30 g
|
|
|
2.
|
How many significant digits are in the following number: 1000 lbs
|
|
|
3.
|
Perform the following calculation. Place your answer in the correct number of
significant digits and in the correct unit : 2.46 cm + 3.8 cm + 15.26 cm
a. | 22 cm | c. | 21.86 cm3 | b. | 21.9 cm | d. | 22.0
cm3 |
|
|
|
4.
|
Perform the following calculation. Place your answer in the correct number of
significant digits and in the correct unit : 16.90 cm ´ 4.60 cm ´ 2.54 cm
a. | 197.46 cm | c. | 197.46 cm3 | b. | 197
cm | d. | 197
cm3 |
|
|
|
5.
|
I decide to build a shelf to hold all of my chemistry books. I need the shelf to
be 4.00 feet long. How many centimeters is that? ( 1 inch = 2.54 cm)
a. | 48 cm | c. | 61 cm | b. | 10.16 cm | d. | 122 cm |
|
|
|
6.
|
One chemical property of matter is
a. | boiling point. | c. | reactivity. | b. | texture. | d. | density. |
|
|
|
7.
|
An example of a chemical change is
a. | sanding wood. | c. | milk
going sour. | b. | melting
ice. | d. | vaporizing gasoline. |
|
|
|
8.
|
A certain atom has 26 protons, 26 electrons, and 30 neutrons. Its mass number is
____.
|
|
|
9.
|
Use the table below to calculate the atomic mass of element X.
Isotope | Mass (amu) | % Abundance | 16X | 15.995 | 99.762% | 17X | 16.999 | 0.038% | 18X | 17.999 | 0.20% | | | |
a. | 15.995 | c. | 17.999 | b. | 16.999 | d. | 15.999 |
|
|
|
10.
|
Which of the following atoms contains the largest number protons?
|
|
|
11.
|
Which two atoms below have the same number of neutrons? 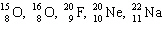
|
|
|
12.
|
What is the identity of 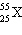 ? a. | zinc | d. | cesium | b. | silver | e. | manganese | c. | iridium |
|
|
|
13.
|
What is the correct Lewis structure for hydrogen chloride, HCl? 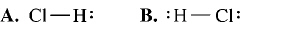 
|
|
|
14.
|
According to VSEPR theory, an AB2 molecule is
a. | trigonal-planar. | c. | linear. | b. | tetrahedral. | d. | octahedral. |
|
|
|
15.
|
According to VSEPR theory, the structure of the ammonia molecule,
NH3, is
a. | trigonal-planar. | c. | trigonal-pyramidal. | b. | bent. | d. | tetrahedral. |
|
|
|
16.
|
What is the correct Lewis structure for ICl 4-? 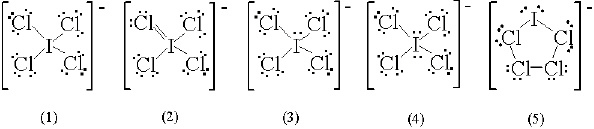
|
|
|
17.
|
The central atom in XeF4 is surrounded by
a. | 3 single bonds, 1 double bond, and no lone pairs of electrons. | b. | 2 single bonds, 2
double bonds, and no lone pairs of electrons. | c. | 3 single bonds, 1 double bond, and 1 lone pair
of electrons. | d. | 4 single bonds, no double bonds, and no lone pairs of electrons. | e. | 4 single bonds, no
double bonds, and 2 lone pairs of electrons. |
|
|
|
18.
|
The correct name for MgCl2 is _______.
a. | magnesium dichloride | d. | magnesium chlorate | b. | magnesium chloride | e. | magnesium perchlorate | c. | magnesium
chlorine |
|
|
|
19.
|
The correct name for N2O5 is _______.
a. | nitrous oxide | d. | nitric oxide | b. | nitrogen pentoxide | e. | nitrogen oxide | c. | dinitrogen
pentoxide |
|
|
|
20.
|
The correct name for H2SO3 is _______.
a. | sulfuric acid | d. | hydrosulfic acid | b. | sulfurous acid | e. | sulfur hydroxide | c. | hydrosulfuric
acid |
|
|
|
21.
|
The formula of hydrobromic acid is __________.
a. | HBr | d. | HBrO3 | b. | HBrO4 | e. | HBrO2 | c. | HBrO |
|
|
|
22.
|
The correct formula of iron(III) bromide is __________.
a. | FeBr2 | d. | Fe3Br3 | b. | FeBr3 | e. | Fe3Br | c. | FeBr |
|
|
|
8 KClO3 +
C12H22O11  8 KCl + 12
CO2 + 11 H2O 8 KCl + 12
CO2 + 11 H2O
|
|
|
23.
|
Using the above reaction, if 25.0 g of carbon dioxide are produced, how many
grams of sugar (C12H22O11) were used?
a. | 0.0473 | c. | 16.2 | b. | 21.1 | d. | 0.0617 |
|
|
|
24.
|
Determine the number of moles in 25.50 g Ag.
a. | 0.236 | c. | 3.64 x
10-4 | b. | 2750 | d. | 4.24 |
|
|
|
25.
|
Write the reaction for the combustion of C4H6, balance the
equation, and determine the coefficient of H2O.
|
|
|
26.
|
How many grams of carbon tetrachloride are in 4.00 moles?
a. | 0.026 | c. | 2.41 x
1024 | b. | 615 | d. | 4.15 x
10-25 |
|
|
|
27.
|
What are the spectator ions in the reaction
between KOH(aq) and HNO3(aq)? a. | K+ and H+ | c. | H+ and
OH- | b. | H+ and OH- | d. | H+ and
NO-3 |
|
|
|
28.
|
Combining aqueous solutions of BaI2 and K2SO4
affords a precipitate of BaSO4. Which ion(s) is/are spectator ions in the
reaction?
a. | Ba2+ only | c. | Ba2+ and SO42- | b. | K+
only | d. | K+ and
I- |
|
|
|
29.
|
How many grams of CH 3Br could be made from 10.0 g of CH 3OH
and excess PBr 3?
3 CH3OH +
PBr3  3
CH3Br + P(OH)3 3
CH3Br + P(OH)3a. | 23.4 | c. | 4.27 | b. | 0.0428 | d. | 0.234 |
|
|
|
30.
|
How many moles are in 1.2 X 1024 molecules of water?
a. | 0.503 | c. | 7.72 x
1047 | b. | 1.38 x 10-48 | d. | 1.99 |
|
|
|
31.
|
A compound contains 36.1 g calcium and 63.9 g
chlorine. What is the empirical formula of the compound?
a. | CaCl3 | c. | CaCl
| b. | Ca2Cl2 | d. | CaCl2 |
|
|
|
32.
|
Determine the empirical formula for a compound that contains 35.98% aluminum and
64.02 % sulfur.
a. | Al3S2 | c. | Al2S3 | b. | Al2S3 | d. | AlS3 |
|
|
|
33.
|
What is the molecular formula of a compound that is
composed of 83.64% tin (Sn) and 16.36% phosphorous (P)? The compound's experimental molar mass
is: 479.98 g/mol.
a. | SnP | c. | SnP4 | b. | Sn3P4 | d. | Sn3P2 |
|
|
|
34.
|
What is the percentage composition of CuCl2?
a. | 33% Cu, 66% Cl | c. | 65.50% Cu, 34.50% Cl | b. | 50% Cu, 50% Cl | d. | 47.267% Cu, 52.733%
Cl |
|
|
|
35.
|
What is the percentage composition of CF4?
a. | 20% C, 80% F | c. | 16.8% C, 83.2% F | b. | 13.6% C, 86.4% F | d. | 81% C, 19% F |
|
|
|
36.
|
An argon ion laser emits light at 488 nm. What is the frequency of this
radiation?
a. | 4.07 ´ 10-19 s-1 | d. | 2.05 ´ 106 s-1 | b. | 1.63 ´ 10-15 s-1 | e. | 6.14 ´
1014 s-1 | c. | 1.46 ´
102 s-1 |
|
|
|
37.
|
What type of orbital is designated n = 4, l = 3,
ml = -3?
|
|
|
38.
|
Which of the following sets of quantum numbers refers to a 3p orbital?
a. | n = 3, 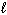 = 0, m = 0, m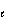 = 0, ms = +
1/2 = 0, ms = +
1/2 | d. | n = 3, 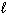 = 3, m = 3, m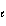 = -2, ms = +
1/2 = -2, ms = +
1/2 | b. | n = 3,  = 1, m = 1, m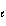 = -1, ms = +
1/2 = -1, ms = +
1/2 | e. | n = 3, 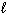 = 3, m = 3, m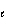 = 3, ms = +
1/2 = 3, ms = +
1/2 | c. | n = 3,  = 2, m = 2, m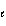 = 1, ms = +
1/2 = 1, ms = +
1/2 |
|
|
|
39.
|
What is the correct orbital diagram for phosphorus? a. | (¯) (¯) (
)( )(
) | b. | (¯) (¯) (¯)( )( ) | c. | (¯) (¯) (¯)(¯)(¯) (¯) (¯)( )( ) | d. | (¯) (¯) (¯)(¯)(¯) (¯) (
)( )(
) | e. | (¯) (¯) (¯)(¯)(¯) (
) (¯)( )(
) |
|
|
|
40.
|
In general, ionization energies
a. | increase down a group and increase across a period. | b. | increase down a
group and decrease across a period. | c. | decrease down a group and increase across a
period. | d. | decrease down a group and decrease across a period. | e. | increase with atomic
mass and increase with atomic radii. |
|
|
|
41.
|
In a solution, the substance that is being dissolved is the ____.
a. | gas | c. | solute | b. | liquid | d. | solvent |
|
|
|
42.
|
How many grams of strontium nitrate Sr(NO3)2 are required
to make 2.00 L of a 0.400M ?
|
|
|
43.
|
What is the concentration (M) of CH3OH in a solution prepared by
dissolving 11.7 g of CH3OH in sufficient water to give 230 mL of solution?
a. | 11.9 | c. | 0.0841 | b. | 1.59 x 10-3 | d. | 1.59 |
|
|
|
44.
|
What volume (mL) of a concentrated solution of sodium hydroxide (6.00 M) must be
diluted to 200 mL to make a 1.50 M solution of sodium hydroxide?
|
|
|
45.
|
The boiling point of a pure substance is _______ than that of a mixture.
a. | lower | c. | the same | b. | higher | d. | has no effect |
|
|
|
46.
|
A 0.225-L flask contains CH4 at 27 °C
and 318 mm Hg. What is the pressure of the CH4 if the volume is increased to 0.500 L and
the temperature increased to 95 °C?
a. | 117 mm Hg | b. | 176 mm Hg | c. | 503 mm
Hg | d. | 508 mm Hg | e. | 867 mm Hg |
|
|
|
47.
|
What volume is occupied by 8.50 g C2H2 at STP (standard
temperature and pressure)? (R = 0.08206 L·atm/mol·K)
a. | 0.670 L | b. | 7.31 L | c. | 7.98
L | d. | 68.7 L | e. | 191 L |
|
|
|
48.
|
Which of the following gases has the greatest density at 25 °C and 5.0 atm?
|
|
|
49.
|
Calculate the density (in g/L) of Kr at 308 K and 527 mm Hg. (R = 0.08206
L·atm/mol·K)
a. | 2.30 g/L | b. | 4.78 g/L | c. | 31.1
g/L | d. | 1.75 ´ 103 g/L | e. | 2.36 ´ 104 g/L |
|
|
|
50.
|
What volume of O2, measured at 91.2 °C and 743 mm Hg, will be produced by the decomposition of 4.88 g
KClO3? (R = 0.08206 L·atm/mol·K)
2 KClO3(s) ® 2 KCl(s) + 3 O2(g)
a. | 0.305 L | b. | 1.22 L | c. | 1.83
L | d. | 24.0 L | e. | 37.4 L |
|
Matching
|
|
|
Classify each of the following as a. | Element | b. | Mixture | c. | Compound |
|
|
|
51.
|
air
|
|
|
52.
|
table salt
|
|
|
Classify each of the following mixtures
as a. | heterogeneous | b. | homogeneous |
|
|
|
53.
|
salt water
|
|
|
Match the terms below with their correct definitions. a. | double-replacement reaction | d. | synthesis
reaction | b. | combustion reaction | e. | decomposition reaction | c. | single-replacement
reaction |
|
|
|
54.
|
A reaction involving the exchange of positive ions between two compounds
dissolved in water
|
|
|
55.
|
A reaction in which two or more substances react to produce a single
product
|