Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
A chemical bond that occurs when atoms share electrons is a(n) ____ bond.
a. | covalent | c. | magnetic | b. | ionic | d. | polyatomic |
|
|
|
2.
|
A compound that is made up only of carbon and hydrogen is called a(n)
____.
a. | alcohol | c. | carbohydrate | b. | amino acid | d. | hydrocarbon |
|
|
|
3.
|
A hydrocarbon that has at least one double or triple bond is ____.
a. | aromatic | c. | substituted | b. | saturated | d. | unsaturated |
|
|
|
4.
|
A hydrocarbon containing only single-bonded carbon atoms is called a(n) ____
hydrocarbon.
a. | aromatic | c. | saturated | b. | substituted | d. | unsaturated |
|
|
|
5.
|
How many electrons does a carbon atom have in its outer energy level?
|
|
|
6.
|
The electrons involved in the formation of a chemical bond are called
a. | dipoles. | c. | Lewis electrons. | b. | s electrons. | d. | valence
electrons. |
|
|
|
7.
|
What are shared in a covalent bond?
a. | ions | c. | electrons | b. | Lewis structures | d. | dipoles |
|
|
|
8.
|
In drawing a Lewis structure, each nonmetal atom except hydrogen should be
surrounded by
a. | 2 electrons. | c. | 8 electrons. | b. | 4 electrons. | d. | 10 electrons. |
|
|
|
9.
|
What is the correct Lewis structure for hydrogen chloride, HCl? 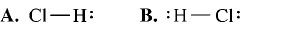 
|
|
|
10.
|
How many atoms of fluorine are present in a molecule of carbon tetrafluoride,
CF4?
|
|
|
11.
|
Name the compound CF4.
a. | calcium fluoride | c. | carbon tetrafluoride | b. | carbon fluoride | d. | monocalcium
quadrafluoride |
|
|
|
12.
|
Name the compound SiO2.
a. | silver oxide | c. | silicon dioxide | b. | silicon oxide | d. | monosilver
dioxide |
|
|
|
13.
|
Name the compound SO3.
a. | sulfur trioxide | c. | selenium trioxide | b. | silver trioxide | d. | sodium trioxide |
|
|
|
14.
|
Name the compound N2O3.
a. | dinitrogen oxide | c. | nitric oxide | b. | nitrogen trioxide | d. | dinitrogen
trioxide |
|
|
|
15.
|
What is the formula for silicon dioxide?
|
|
|
16.
|
What is the formula for dinitrogen trioxide?
|
|
|
17.
|
What is the formula for sulfur dichloride?
|
|
|
18.
|
What is the formula for diphosphorus pentoxide?
a. | P2PeO5 | c. | P2O4 | b. | PO5 | d. | P2O5 |
|
|
|
19.
|
What is the formula for hydrochloric acid?
|
|
|
20.
|
Carbon shows a very strong tendency to form
a. | ionic bonds. | c. | hydrogen bonds. | b. | covalent bonds. | d. | highly polar
bonds. |
|
|
|
21.
|
How many covalent bonds can a carbon atom usually form?
|
|
|
22.
|
What do all organic compounds contain?
a. | hydrogen | c. | oxygen | b. | water | d. | carbon |
|
|
|
23.
|
Organic compounds are defined as all covalently bonded compounds containing
carbon except
a. | oxides and carbonates. | c. | ethers and esters. | b. | alcohols and acids. | d. | aldehydes and
ketones. |
|
|
|
24.
|
Which hydrocarbons have six-membered carbon rings and delocalized
electrons?
a. | alkanes | c. | alkynes | b. | alkenes | d. | aromatic
hydrocarbons |
|
|
|
25.
|
Each carbon atom in a molecule forms four single covalent bonds with other atoms
in a(n)
a. | aromatic hydrocarbon. | c. | structural isomer. | b. | saturated hydrocarbon. | d. | geometric
isomer. |
|
|
|
26.
|
Hydrocarbons in which carbon atoms are connected by only single bonds in
straight chains or branched chains are called
a. | aromatic hydrocarbons. | c. | alkenes. | b. | alkynes. | d. | alkanes. |
|
|
|
27.
|
Which of the following is an alkane?
a. | propyne | c. | propene | b. | propane | d. | propyl bromide |
|
|
|
28.
|
Which of the following is an atom or a group of atoms responsible for the
specific properties of an organic compound?
a. | isomer | c. | substituted hydrocarbon | b. | hydrocarbon | d. | functional group |
|
|
|
29.
|
What is the functional group in carboxylic acids?
|
|
|
30.
|
What is the name of the functional group –OH?
a. | hydroxyl group | c. | carboxyl group | b. | amine group | d. | hydroxide ion |
|
|
|
31.
|
What is the formula of the carboxyl group?
|
|
|
32.
|
Which of these elements does not exist as a diatomic molecule?
|
|
|
33.
|
How do atoms achieve noble-gas electron configurations in single covalent
bonds?
a. | One atom completely loses two electrons to the other atom in the
bond. | b. | Two atoms share two pairs of electrons. | c. | Two atoms share two
electrons. | d. | Two atoms share one electron. |
|
|
|
34.
|
Which of the following elements can form diatomic molecules held together by
triple covalent bonds?
a. | carbon | c. | fluorine | b. | oxygen | d. | nitrogen |
|
|
|
35.
|
Which of the following diatomic molecules is joined by a double covalent
bond?
|
|
|
36.
|
Sulfur hexafluoride is an example of a ____.
a. | monatomic ion | c. | binary compound | b. | polyatomic ion | d. | polyatomic
compound |
|
|
|
37.
|
What is the ending for the names of all binary compounds, both ionic and
molecular?
|
|
|
38.
|
Binary molecular compounds are made of two ____.
a. | metallic elements | c. | polyatomic ions | b. | nonmetallic elements | d. | cations |
|
|
|
39.
|
In naming a binary molecular compound, the number of atoms of each element
present in the molecule is indicated by ____.
a. | Roman numerals | c. | prefixes | b. | superscripts | d. | suffixes |
|
|
|
40.
|
Which of the following correctly shows a prefix used in naming binary molecular
compounds with its corresponding number?
a. | deca-, 7 | c. | hexa-, 8 | b. | nona-, 9 | d. | octa-, 4 |
|
|
|
41.
|
Which of the following is a binary molecular compound?
a. | BeHCO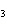 | c. | AgI | b. | PCl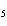 | d. | MgS |
|
|
|
42.
|
When naming acids, the prefix hydro- is used when the name of the acid
anion ends in ____.
|
|
|
43.
|
Which of the following shows both the correct formula and correct name of an
acid?
a. | HClO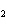 , chloric acid , chloric acid | c. | H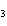 PO PO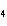 , phosphoric acid , phosphoric acid | b. | HNO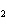 , hydronitrous
acid , hydronitrous
acid | d. | HI, iodic
acid |
|
|
|
44.
|
What is the name of H 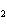 SO 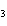 ? a. | hyposulfuric acid | c. | sulfuric acid | b. | hydrosulfuric acid | d. | sulfurous acid |
|
|
|
45.
|
When the name of an anion that is part of an acid ends in -ite, the acid
name includes the suffix ____.
|
|
|
46.
|
What is the formula for sulfurous acid?
|
|
|
47.
|
What is the formula for phosphoric acid?
|
|
|
48.
|
What is the formula for hydrosulfuric acid?
|
|
|
49.
|
Select the correct formula for sulfur hexafluoride.
|
|
|
50.
|
Suppose you encounter a chemical formula with H as the cation. What do you know
about this compound immediately?
a. | It is a polyatomic ionic compound. | c. | It is a base. | b. | It is an
acid. | d. | It has a 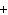 1
charge. 1
charge. |
|