Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
In a chemical formula, the number of each type of atom in the compound is shown
by numbers called ____.
a. | superscripts | c. | oxidation numbers | b. | chemical symbols | d. | subscripts |
|
|
|
2.
|
A group of covalently bonded atoms that acts together as one charged atom is a
____.
a. | crystal | c. | negative ion | b. | molecule | d. | polyatomic ion |
|
|
|
3.
|
The sum of the oxidation numbers in a neutral compound is always ____.
a. | a negative number | c. | a positive number | b. | one | d. | zero |
|
|
|
4.
|
What is the name of the compound with the formula NaCl?
a. | chlorine sodiate | c. | sodium chloride | b. | sodium chlorate | d. | sodium
dichloride |
|
|
|
5.
|
Which of the following is the correct formula for magnesium nitrate?
a. | MgNO3 | c. | Mg(NO3)2 | b. | Mg2NO3 | d. | Mg2(NO3)2 |
|
|
|
6.
|
What is the charge of phosphate in K3PO4?
|
|
|
7.
|
What is the correct name for K2SO4?
a. | potassium disulfide | c. | potassium sulfide | b. | potassium sulfate | d. | potassium(II)
sulfate |
|
|
|
8.
|
What is the correct formula for magnesium oxide?
|
|
|
9.
|
What is the formula for zinc fluoride?
|
|
|
10.
|
What is the formula for the compound formed by calcium ions and chloride
ions?
a. | CaCl | c. | CaCl3 | b. | Ca2Cl | d. | CaCl2 |
|
|
|
11.
|
What is the formula for the compound formed by lead(II) ions and chromate
ions?
a. | PbCrO4 | c. | Pb2(CrO4)3 | b. | Pb2CrO4 | d. | Pb(CrO4)2 |
|
|
|
12.
|
What is the formula for aluminum sulfate?
a. | AlSO4 | c. | Al2(SO4)3 | b. | Al2SO4 | d. | Al(SO4)3 |
|
|
|
13.
|
What is the formula for barium hydroxide?
a. | BaOH | c. | Ba(OH)2 | b. | BaOH2 | d. | Ba(OH) |
|
|
|
14.
|
Name the compound Ni(ClO3)2.
a. | nickel(II) chlorate | c. | nickel(II) chlorite | b. | nickel(II) chloride | d. | nickel(II)
peroxide |
|
|
|
15.
|
Name the compound Zn3(PO4)2.
a. | zinc potassium oxide | c. | zinc phosphate | b. | trizinc polyoxide | d. | zinc phosphite |
|
|
|
16.
|
Name the compound KClO3.
a. | potassium chloride | c. | potassium chlorate | b. | potassium trioxychlorite | d. | hypochlorite |
|
|
|
17.
|
Name the compound Fe(NO3)2.
a. | iron(II) nitrate | c. | iron(III) nitrate | b. | iron(II) nitrite | d. | iron(III)
nitride |
|
|
|
18.
|
Name the compound Al2S3.
a. | aluminum sulfate | c. | aluminum(II) sulfate | b. | aluminum sulfur | d. | aluminum
sulfide |
|
|
|
19.
|
What is the oxidation number of oxygen in most compounds?
|
|
|
20.
|
What is the oxidation number of hydrogen in H2O?
|
|
|
21.
|
Which atom is most likely to form a 1- ion?
|
|
|
22.
|
Which atom is most likely to form a 3+ ion?
|
|
|
23.
|
What charge is likely on a monatomic silver cation?
|
|
|
24.
|
For a nonmetal in Group 5A of the periodic table, the most common monatomic ion
will have a charge of ____.
|
|
|
25.
|
What is the correct formula for an ionic compound that contains aluminum ions
and carbonate ions?
a. | AlCO3 | b. | Al(CO3)2 | c. | Al(CO3)3 | d. | Al2(CO3)3 | e. | Al3(CO3)2 |
|
|
|
26.
|
What is the correct formula for an ionic compound that contains magnesium ions
and fluoride ions?
a. | Mg2F | b. | MgF | c. | Mg2F2 | d. | MgF2 | e. | Mg2F3 |
|
|
|
27.
|
What is the charge on the lead ion in PbS2?
|
|
|
28.
|
What is the charge on the copper ion in Cu2O?
|
|
|
29.
|
What is the correct formula for sodium acetate?
a. | Na2CH3O | b. | NaCH3CO2 | c. | NaCH3O | d. | NaCH2O2 | e. | NaCHO |
|
|
|
30.
|
What is the correct formula for barium nitrate?
a. | Ba(NO3)2 | b. | BNO2 | c. | Ba(NO2)2 | d. | BaN | e. | BaNO3 |
|
|
|
31.
|
What is the correct formula for cobalt(III) oxide?
a. | CoO | b. | Co3O | c. | Co3O2 | d. | Co2O3 | e. | CoO3 |
|
|
|
32.
|
What is the correct formula for copper(II) chloride?
a. | Cu2Cl | b. | CuCl | c. | CuCl2 | d. | CuCl3 | e. | Cu2Cl2 |
|
|
|
33.
|
What is the correct formula for vanadium(V) sulfide?
a. | V2S5 | b. | V5S | c. | VS5 | d. | V10S5 | e. | V5S2 |
|
|
|
34.
|
What is the correct name for NH4ClO4?
a. | ammonia hydrogen chlorate | b. | ammonia hydrogen
perchlorate | c. | ammonium perchlorate | d. | ammonium hypochlorite | e. | ammonium
hydrochloric acid |
|
|
|
35.
|
What is the correct name for AlBr3?
a. | aluminum tribromium | b. | aluminum tribromide | c. | aluminum(III)
tribromide | d. | aluminum bromide | e. | bromine
aluminide |
|
|
|
36.
|
What is the correct name for Cr(NO3)2?
a. | chromium(II) nitrate | b. | chromium(II) dinitrate | c. | chromium
dinitrate | d. | dinitrochromate | e. | chromium(II)
nitride |
|
|
|
37.
|
What is the formula of sodium bicarbonate, or baking soda?
a. | CH2ONa | b. | NaHCO3 | c. | N2CO3 | d. | Na2CO3 | e. | H2CO3 |
|
|
|
38.
|
How many valence electrons are in an atom of phosphorus?
|
|
|
39.
|
How many valence electrons are in an atom of magnesium?
|
|
|
40.
|
How many valence electrons does a helium atom have?
|
|
|
41.
|
Which of the following elements does NOT form an ion with a charge of 1 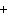 ? a. | fluorine | c. | potassium | b. | hydrogen | d. | sodium |
|
|
|
42.
|
How many electrons does nitrogen gain in order to achieve a noble-gas electron
configuration?
|
|
|
43.
|
How does oxygen obey the octet rule when reacting to form compounds?
a. | It gains electrons. | b. | It gives up electrons. | c. | It does not change
its number of electrons. | d. | Oxygen does not obey the octet
rule. |
|
|
|
44.
|
What is the charge on the cation in the ionic compound sodium sulfide?
|
|
|
45.
|
Which of the following occurs in an ionic bond?
a. | Oppositely charged ions attract. | b. | Two atoms share two
electrons. | c. | Two atoms share more than two electrons. | d. | Like-charged ions
attract. |
|
|
|
46.
|
What is the net charge of the ionic compound calcium fluoride?
a. | 2– | c. | 0 | b. | 1– | d. | 1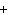 |
|
|
|
47.
|
What is the formula unit of sodium nitride?
|
|
|
48.
|
What is the formula unit of aluminum oxide?
|
|
|
49.
|
What is the name of the ionic compound formed from lithium and bromine?
a. | lithium bromine | c. | lithium bromium | b. | lithium bromide | d. | lithium bromate |
|
|
|
50.
|
What is the formula for sodium sulfate?
|