True/False
Indicate whether the sentence or statement is true
or false.
|
|
|
1.
|
In
one mole of freon, the chemical ratio of carbon to chlorine to fluorine is 2:1:2.
|
|
|
2.
|
The
percent by mass of each element in a compound is known as the percent composition of a
compound.
|
|
|
3.
|
The
molecular formula for a compound is the formula with the smallest whole-number mole ratio of the
elements.
|
Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
4.
|
A
mole of potassium chloride (KCl) contains 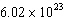 _____. _____. a. | atoms
KCl. | c. | ions
KCl. | b. | formula units
KCl. | d. | molecules
KCl. | | | | |
|
|
|
5.
|
The
SI unit of molar mass is the _____. a. | gram. | c. | mole. | b. | gram/mole. | d. | mole/gram. | | | | |
|
|
|
6.
|
Which
conversion factor would you use to calculate correctly the mass of 2 moles of the element
titanium?
|
|
|
7.
|
How
many moles of oxygen atoms do 1.5 moles of CO2 contain? a. | 1
mol | c. | 2
mol | b. | 1.5
mol | d. | 3.0
mol | | | | |
|
|
|
8.
|
Which
compound has the smallest molar mass?
|
|
|
9.
|
One
mole of silicon (Si) has a mass of 28.086 g, and one mole of carbon has a mass of 12.011 g. What is
the mass of one mole of silicon carbide (SiC)? a. | 2.340 g | b. | 16.075
g | c. | 40.097
g | d. | 3.3734 ´
102 g | | |
|
|
|
10.
|
Methane (CH4) contains 75% carbon. What percentage of methane is
hydrogen?
|
|
|
11.
|
Which
information about a compound can you use to begin to determine the empirical and molecular formulas
of the compound? a. | mass of the
compound | c. | percent
composition of the compound | b. | number of elements in the
compound | d. | volume of the
compound | | | | |
|
|
|
12.
|
You
have determined that a compound is composed of 0.300 moles of carbon and 0.600 moles of oxygen. What
must you do to determine the mole ratio of the elements in the empirical formula of the
compound? a. | Multiply each
mole value by 0.300 mol. | c. | Divide each mole
value by 0.300 mol. | b. | Multiply each mole value by 0.600
mol. | d. | Divide each mole
value by 0.600 mol. | | | | |
|
|
|
13.
|
The
mole ratio of carbon to hydrogen to oxygen in a compound is 1 mol C : 2 mol H : 1 mol O. What is the
empirical formula of the compound? a. | CHO | c. | C2HO2 | b. | CH2O | d. | C2H2O2 | | | | |
|
|
|
14.
|
You
calculate the mole ratio of oxygen to aluminum in a compound to be 1.5 mol O : 1 mol Al. What should
you do to determine the mole ratio in the empirical formula of the compound? a. | Multiply each
mole value by 1.5. | c. | Divide each mole
value by 1.5. | b. | Multiply each mole value by 2. | d. | Divide each mole value by 2. | | | | |
|
|
|
15.
|
What
is the relationship between the molecular formula and the empirical formula of a
compound? a. | (molecular
formula)(empirical formula) = n
| b. | molecular formula = 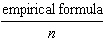 | c. | molecular
formula = (empirical formula)n
| d. | molecular formula = | | |
|
|
|
16.
|
You
know that the empirical formula of a compound has a molar mass of 30.0 g/mol. The experimental molar
mass of this compound is 60.0 g/mol. What must you do to determine the value of n in the relationship
between the molecular formula and the empirical formula? a. | Add 30.0 g/mol
and 60.0 g/mol. | c. | Divide 60.0
g/mol by 30.0 g/mol. | b. | Divide 30.0 g/mol by 60.0
g/mol. | d. | Multiply 30.0
g/mol by 60.0 g/mol. | | | | |
|
|
|
17.
|
You
know that the experimental molar mass of a compound is three times the molar mass of its empirical
formula. If the compound’s empirical formula is NO2, what is its molecular
formula?
|
|
|
18.
|
What
is the SI base unit used to measure the amount of a substance? a. | Kelvin | c. | Meter | b. | Kilogram | d. | Mole | | | | |
|
|
|
19.
|
Calculate the number of molecules in 4.0 mol H2O. a. | 0.60 x
1023 molecules | c. | 2.4 x
10–23 molecules | b. | 2.4 x 1024 molecules | d. | 2.4 x 1023 molecules | | | | |
|
|
|
20.
|
How
many moles of Ag contain 4.49 ´ 1023 atoms Ag? a. | 0.745 x
1024 mol | c. | 0.745
mol | b. | 0.745 x
1023 mol | d. | 27.0
mol | | | | |
|
|
|
21.
|
Calculate the number of atoms in 13.2 mol copper. a. | 2.19 x
1023 atoms | c. | 7.95 x
10–23 atoms | b. | 7.95 x 1024 atoms | d. | 79.5 x 1023 atoms | | | | |
|
|
|
22.
|
Copper (Cu) is a transition element used in the making of coins. Calculate the mass in
grams of 0.0420 moles of copper. a. | 0.00697 g | c. | 2.67 g | b. | 0.252
g | d. | 6.61
g | | | | |
|
|
|
23.
|
Determine the mass in grams of 0.0489 mol cobalt. a. | 0.00812
g | c. | 2.88
g | b. | 0.294
g | d. | 0.000830
g | | | | |
|
|
|
24.
|
How
many moles of calcium are in 425 g calcium (Ca)? a. | 10.6 mol | c. | 171 mol | b. | 70.5
mol | d. | 255
mol | | | | |
|
|
|
25.
|
Copper is one of a group of metals called the coinage metals. How many atoms of copper
(Cu) are in a pure copper coin weighing 12.0 g? a. | 0.0313 x 1023 atoms | c. | 1.10 x 1023 atoms | b. | 0.187 x
1023 atoms | d. | 1.13 x
1023 atoms | | | | |
|
|
|
26.
|
A
balloon contains 4.50 ´ 1022 atoms of helium (He) gas. Calculate the mass of helium
in grams. a. | 0.185
g | c. | 0.0747
g | b. | 0.0185
g | d. | 0.299
g | | | | |
|
|
|
27.
|
What
is the molar mass of Ca(OH)2? a. | 57.008 g/mol | c. | 73.088 g/mol | b. | 73.080
g/mol | d. | 74.092
g/mol | | | | |
|
|
|
28.
|
What
is the mass of 2.25 moles of sulfuric acid (H2SO4)? a. | 50.0
g | c. | 112
g | b. | 98.0
g | d. | 220
g | | | | |
|
|
|
29.
|
How
many grams of potassium permanganate are in 2.20 moles? a. | 53.2
g | c. | 242
g | b. | 158
g | d. | 347
g | | | | |
|
|
|
30.
|
Determine the number of moles present in 32.5 g aluminum chloride. a. | 0.244
mol | c. | 1.21
mol | b. | 4.10
mol | d. | 720
mol | | | | |
|
|
|
31.
|
What
is the mass in grams of 1.02 x 1024 atoms manganese (Mn)? a. | 0.112 x
101 g | c. | 9.30 x
10–1 g | b. | 0.169 x 101 g | d. | 9.30 x 101 g | | | | |
|
|
|
32.
|
How
many moles are present in 21.2 g hydrochloric acid? a. | 0.582
mol | c. | 21.0
mol | b. | 1.72
mol | d. | 128
mol | | | | |
|
|
|
33.
|
The
molecular mass of potassium dichromate, K2Cr2O7, is
__________. a. | 107.09 | d. | 294.18 | b. | 255.08 | e. | 333.08 | c. | 242.18 | | | | |
|
|
|
34.
|
The
molecular mass of the ionic compound (NH4)2SO4 is
__________. a. | 100 | d. | 132 | b. | 118 | e. | 264 | c. | 116 | | | | |
|
|
|
35.
|
What
is the mass % of carbon in dimethylsulfoxide, C2H6SO? a. | 60.0 | d. | 7.74 | b. | 20.6 | e. | 79.8 | c. | 30.7 | | | | |
|
|
|
36.
|
The
mass % of F in the binary compound KrF2 is __________. a. | 18.48 | d. | 81.52 | b. | 145.3 | e. | 31.20 | c. | 68.80 | | | | |
|
|
|
37.
|
A
compound was found to contain 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen. What is the
empirical formula of the compound? a. | CH2O | d. | C3H6O | b. | C6HO8 | e. | C4H7O53 | c. | C2H4O2 | | | | |
|
|
|
38.
|
A
compound containing only carbon and hydrogen is 85.7% by mass carbon and 14.3% by mass
hydrogen. What is the empirical formula of the compound? a. | CH2 | d. | C4H8 | b. | C2H4 | e. | C86H14 | c. | CH4 | | | | |
|
Matching
|
|
|
Match the terms below with their correct definitions. a. | Avogadro’s
number | e. | mole | b. | empirical formula | f. | molecular formula | c. | hydrate | g. | percent
composition | d. | molar mass | | | | |
|
|
|
39.
|
Percent by mass of each element in a compound
|
|
|
40.
|
Mass
in grams of one mole of any pure substance
|
|
|
41.
|
Formula of a compound with the smallest whole-number mole ratio of the
elements
|
|
|
42.
|
Specifies the actual number of atoms of each element in one molecule of a
compound
|
|
|
43.
|
SI
base unit used to measure the amount of a substance
|
|
|
44.
|
|
|
|
Match the letter of the conversion factor that is needed to solve the problem. You
may need to use more than one conversion factor to solve the problem.
|
|
|
45.
|
Find
the number of moles in 23.0 g of zinc.
|
|
|
46.
|
Find
the mass of 5.0 ´ 1020 zinc atoms.
|
|
|
47.
|
Find
the mass of 2.00 moles of zinc.
|
|
|
48.
|
Find
the number of atoms in 7.40 g of zinc.
|
|
|
49.
|
Find
the number of moles that contain 4.25 ´ 1027 zinc atoms.
|
|
|
50.
|
Find
the number of atoms in 3.25 moles of zinc.
|