Multiple Choice
Identify the
letter of the choice that best completes the statement or answers the question.
|
|
|
1.
|
If
hydrochloric acid and potassium hydroxide react, what is the product of the net ionic equation for
the reaction? a. | hydrochloric
acid | c. | potassium
chloride | b. | hydrogen ions | d. | water | | | | |
|
|
|
2.
|
H+(aq) +
Br–(aq) + K+(aq) + OH2(aq) 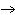 H2O(l) + Br–(aq) + K+(aq) is an example of what type of
chemical equation?
H2O(l) + Br–(aq) + K+(aq) is an example of what type of
chemical equation? a. | complete ionic | c. | precipitation | b. | net
ionic | d. | spectator | | | | |
|
|
|
3.
|
What
type of reaction takes place when fluorine reacts with sodium bromide? a. | Single-replacement | c. | Combination | b. | Double-replacement | d. | Decomposition | | | | |
|
|
|
4.
|
What
are the products of the double-replacement reaction between aqueous hydrogen bromide and aqueous
sodium hydroxide? a. | Sodium
oxybromide and water | c. | Sodium hydro
bromide and hydroxyl ions | b. | Sodium hydride, bromine, and
water | d. | Water and sodium
bromide | | | | |
|
|
|
5.
|
Which
of the following is a balanced chemical equation? a. | AgNO3 + NaCl ® 4AgCl +
2NaNO3 | b. | 2AgNO3 + 2NaCl ® 3AgCl +
2NaNO3 | c. | AgNO3 + NaCl ® AgCl +
NaNO3 | d. | AgNO3 + 2NaCl ® AgCl +
3NaNO3 | | |
|
|
|
6.
|
Which
of the following could represent a decomposition reaction? a. | compound =
element + element | b. | compound + compound = compound +
compound | c. | element + compound = element +
compound | d. | element + element = compound | | |
|
|
|
7.
|
According to the law of conservation of mass, how does the mass of the products in a
chemical reaction compare to the mass of the reactants? a. | There is no
relationship. | b. | The mass of the products is greater. | c. | The mass of the
reactants is greater. | d. | The masses are equal. | | |
|
|
|
8.
|
What
is an insoluble compound that forms during a chemical reaction? a. | aqueous | c. | inhibitor | b. | catalyst | d. | precipitate | | | | |
|
|
|
9.
|
A
chemical reaction in which two or more substances combine to form another substance is called a
____. a. | synthesis
reaction | c. | product | b. | decomposition reaction | d. | reactant | | | | |
|
|
|
10.
|
According to the law of conservation of mass, if two atoms of hydrogen are used as a
reactant, how many atoms of hydrogen must be part of the product?
|
|
|
11.
|
What
type of reaction is shown in the following chemical equation: 2H2O ® 2H2
+ O2? a. | decomposition | c. | single–displacement | b. | double–displacement | d. | synthesis | | | | |
|
|
|
12.
|
The
formula for the products between sodium hydroxide and sulfuric acid are ___. a. | NaSO4
+ H2O | c. | NaS2
+ H2O | b. | Na2SO4 +
H2O | d. | NaH +
SOH | | | | |
|
|
|
13.
|
Given
the following combustion reaction of a hydrocarbon, balance the equation and determine the
coefficient of H2O.
C4H6 + O2 ®
CO2 + H2O
|
|
|
14.
|
In
the equation 2Fe + 3F2 ® 2FeF3, what is
iron? a. | product | c. | acid | b. | reactant | d. | base | | | | |
|
|
|
15.
|
Mole
ratios for a reaction are obtained from what? a. | Avogadro’s number | c. | subscripts | b. | balanced
chemical equation | d. | charges | | | | |
|
|
|
16.
|
What
is the coefficient that belongs in front of carbon dioxide in the
equation?
____C3H8 + ___O2 ®
____CO2 + ___H2O
|
|
|
17.
|
The following reaction is an
example of which type of reaction?
AgNO3(aq) + NaCl (aq) ® AgCl(s) +
NaNO3(aq) a. | synthesis | c. | single replacement | b. | decomposition | d. | double
replacement | | | | |
|
|
|
18.
|
What
are the coefficients to correctly balance the formula equation
Pb(NO3)2 (aq) + KI (aq) ®
PbI2 (s) + KNO3(aq) a. | 1 2 1
2 | c. | 1 4 1
4 | b. | 2 1 2
1 | d. | 4 1 4
1 | | | | |
|
|
|
19.
|
What
is the balanced equation when aluminum reacts with copper(II)sulfate? a. | Al +
CuSO4 ® Cu + AlSO4 | c. | 4Al + 3Cu2(SO4)2 ® 6Cu +
2Al2(SO4)3 | b. | 2Al +
3CuSO4 ® 3Cu +
Al2(SO4)3 | d. | 2Al + Cu2(SO4)2 ® 2Cu +
2AlSO4 | | | | |
|
|
|
20.
|
How
do you know when a chemical equation is balanced?. a. | the number of
atoms on the left equals the number of atoms on the right | c. | the product has less grams | b. | the total number
of moles of reactants equals the total number of moles of product | d. | the product has more grms | | | | |
|
|
|
21.
|
Write
the reaction for the combustion of C4H6, balance the equation, and determine
the coefficient of H2O.
|
|
|
22.
|
The
spectator ions in the reaction between aqueous perchloric acid and aqueous barium hydroxide are
____________. a. | OH-
and ClO4- | c. | H+
and OH- | b. | H+, OH-, ClO4-, and
Ba2+ | d. | ClO4- and Ba2+ | | | | |
|
|
|
23.
|
Consider the following reaction:
Al(NO3)3 + Na2S ®
Al2S3 + NaNO3 a. | 2,3,1,6 | c. | 1,1,1,1 | b. | 2,1,3,2 | d. | 4,6,3,2 | | | | |
|
|
|
24.
|
What
is the coefficient of Al when the following equation is balanced?
Al +
H2O ® Al(OH)3 + H2
|
|
|
25.
|
What is the coefficient of H2S when the following
equation is balanced?
FeCl3 + H2S ® Fe2S3 + HCl
|
|
|
26.
|
What is the coefficient of FeCl3 when the following
equation is balanced?
FeCl3 + Na2CO3 ® Fe2(CO3)3 +
NaCl
|
Matching
|
|
|
Match the
terms below with their correct definitions. a. | aqueous solution | j. | precipitate | b. | chemical equation | k. | product | c. | chemical reaction | l. | reactant | d. | coefficient | m. | single-replacement reaction | e. | combustion
reaction | n. | spectator
ion | f. | complete ionic
equation | o. | synthesis
reaction | g. | decomposition reaction | p. | solute | h. | double-replacement reaction | q. | solvent | i. | net ionic equation | | | | |
|
|
|
27.
|
A reaction
in which a compound breaks down into two or more elements or new compounds
|
|
|
28.
|
A number
written in front of a chemical formula
|
|
|
29.
|
A solid
produced during a chemical reaction in a solution
|
|
|
30.
|
A solution
in which the solvent is water
|
|
|
31.
|
A statement
that uses chemical formulas to show the identities and relative amounts of the substances involved in
a chemical reaction
|
|
|
32.
|
An equation
that shows all of the particles in solution as they actually exist
|
|
|
33.
|
An equation
that includes only the particles that participate in the reaction
|
|
|
34.
|
An ion that
is present but does not participate in a reaction
|
|
|
35.
|
A reaction
in which oxygen combines with a substance and releases heat and light energy
|
|
|
36.
|
A reaction
in which the atoms of one element replace the atoms of another element in a compound
|
|
|
37.
|
A reaction
involving the exchange of positive ions between two compounds dissolved in water
|
|
|
38.
|
The process
by which the atoms of one or more substances are rearranged to form different
substances
|
|
|
39.
|
A starting
substance in a chemical reaction
|
|
|
40.
|
A substance
formed during a chemical reaction
|
|
|
41.
|
A reaction
in which two or more substances react to produce a single product
|
|
|
Assume
that Q, T, X, and Z are symbols for elements. Match each equation with the reaction type it
represents a. | decomposition | c. | single-replacement | b. | double-replacement | d. | synthesis | | | | |
|
|
|
42.
|
Q + XZ 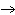 X + QZ X + QZ
|
|
|
43.
|
Q + Z 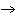 QZ QZ
|
|
|
44.
|
QT 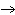 Q + T Q + T
|
|
|
45.
|
QT + XZ 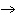 QZ + XT QZ + XT
|
|
|
a. | precipitate | d. | coefficient | b. | product | e. | spectator ion | c. | reactant | | | | |
|
|
|
46.
|
A
number written in front of a chemical reaction
|
|
|
47.
|
A
solid produced during a chamical reaction
|
|
|
48.
|
An
ion that is present, but does not participate in the reaction
|
|
|
49.
|
A
substance formed during a chemical reaction
|
|
|
50.
|
A
starting substance in a chemical reaction
|